Watch It Work
See how pharma training videos become FDA-compliant documentation instantly
GMP training videos become FDA-compliant, auditable documentation
FDA/GMP Compliance Features
Docsie generates training documentation that meets FDA CFR 21 Part 11 requirements—with audit trails, version control, and GMP procedure formatting.
Generate training documentation with audit trails, version history, and change control that meets FDA electronic records requirements for pharmaceutical training
AI recognizes Good Manufacturing Practice terminology and structures content as SOPs, batch records, and quality procedures—not generic documentation
Create documented evidence of training completion with timestamps and content traceability—ready for FDA inspections and regulatory audits
Simple Process
Powered by Docsie Copilot's compliance-aware AI
Upload your GMP training, quality assurance videos, or regulatory training recordings from your LMS or training library
AI extracts procedures, quality parameters, and regulatory requirements—structures content for FDA and GMP compliance
Get documented training procedures with version control and audit trails that meet FDA CFR 21 Part 11 requirements
See how pharmaceutical companies transform GMP training into FDA-compliant documentation

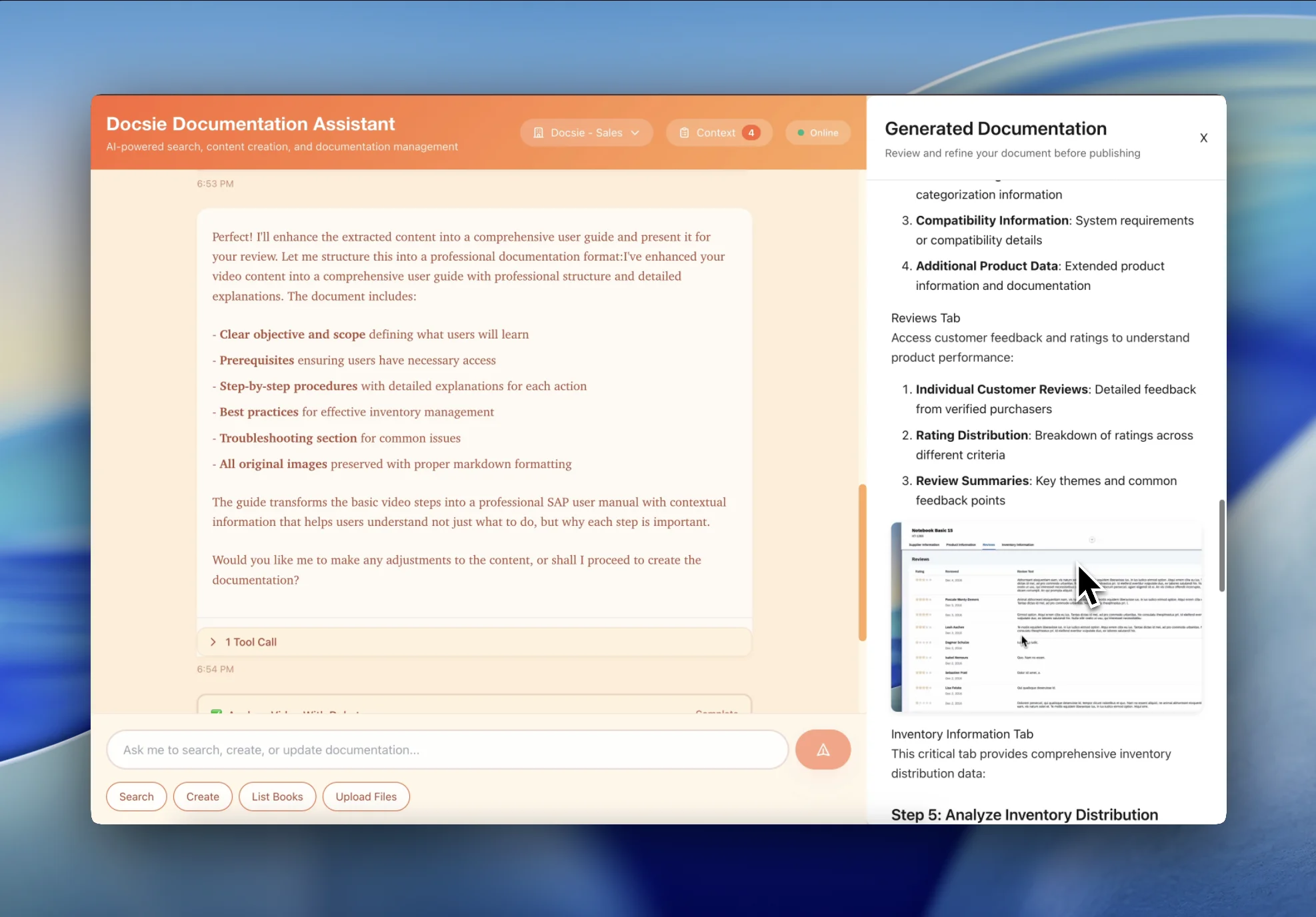
Transform hours of Good Manufacturing Practice training into searchable, version-controlled SOPs that meet FDA documentation requirements. Perfect for pharmaceutical manufacturing, quality assurance, and regulatory compliance.

Turn quality assurance training videos into structured documentation with validation protocols, inspection procedures, and deviation handling guidelines ready for regulatory audits.

Convert FDA regulation training, ICH guideline videos, and regulatory procedure training into organized, searchable compliance knowledge base with traceability to source training.
Everything you need to convert pharma training videos into FDA-compliant documentation
Generate documentation with timestamps, version control, and audit trails that support FDA regulatory requirements
Automatically identify and structure Good Manufacturing Practice procedures, critical process parameters, and quality control points
Convert training videos into documentation in multiple languages for global manufacturing sites and regulatory submissions
Generate searchable documentation with automatic linking to FDA regulations, ICH guidelines, and internal SOPs
Automatically capture critical visual evidence of equipment setup, manufacturing processes, and quality checks
Structure documentation to support IQ/OQ/PQ validation with step-by-step procedures and acceptance criteria
Watch how Docsie Copilot analyzes both audio and video—seeing UI elements, reading on-screen text, and capturing code—to create structured documentation
No credit card required • 14-day free trial
Common Questions
Everything you need to know about converting pharma training to FDA-compliant documentation
Q: Can I use this for FDA-regulated documentation and GMP training?
A: Yes. Docsie generates documentation with audit trails, version control, and timestamps that support FDA regulatory requirements. The system maintains a complete record of all changes and helps create documented evidence of training completion for regulatory submissions.
Q: Can I use this for GMP training documentation required by FDA?
A: Absolutely. Docsie converts GMP training videos into written procedures, SOPs, and training records that FDA auditors expect to see. The system automatically structures content to match GMP documentation requirements and maintains traceability to the original training video.
Q: How does this handle validation protocols (IQ/OQ/PQ)?
A: Docsie's AI recognizes validation terminology and automatically structures Installation Qualification, Operational Qualification, and Performance Qualification procedures. It captures acceptance criteria, test procedures, and results documentation in a format suitable for validation packages.
Q: Does the AI understand pharmaceutical terminology and GMP concepts?
A: Yes. Our multimodal AI is trained on pharmaceutical and regulatory content. It recognizes GMP terminology, regulatory citations (21 CFR, ICH guidelines), process validation concepts, and pharmaceutical manufacturing procedures—ensuring accurate documentation that doesn't misinterpret critical compliance terms.
Q: Can I customize the documentation format for our QMS?
A: Yes. The AI-generated documentation serves as a structured first draft that you can edit and format to match your Quality Management System requirements. All SOP numbering, formatting, and approval workflows can be customized while maintaining the core content integrity.
Still have questions?
Book a DemoCompatible with major video platforms and formats

Process YouTube videos and playlists

Convert Vimeo content
Support for MP4, AVI, WebM, MOV
Start creating professional documentation that your users will love